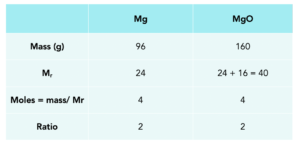
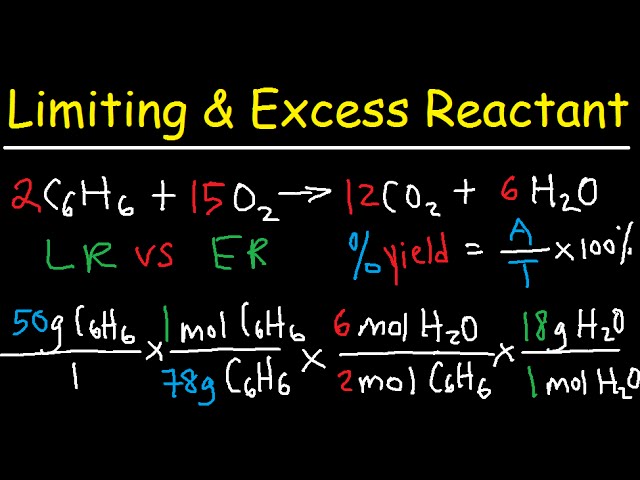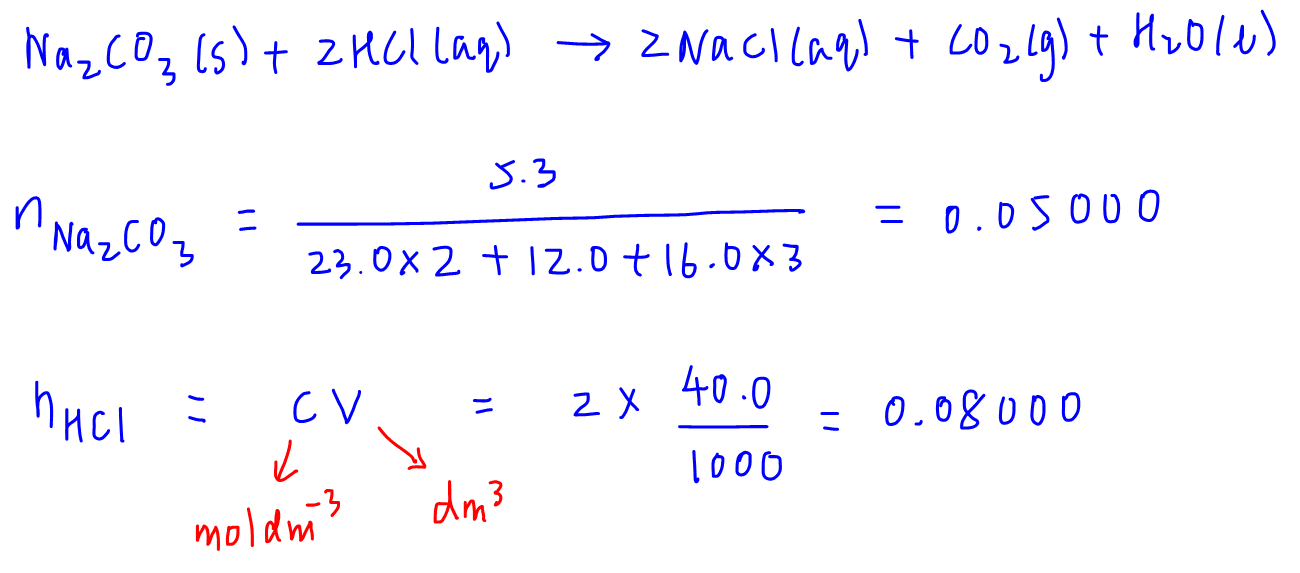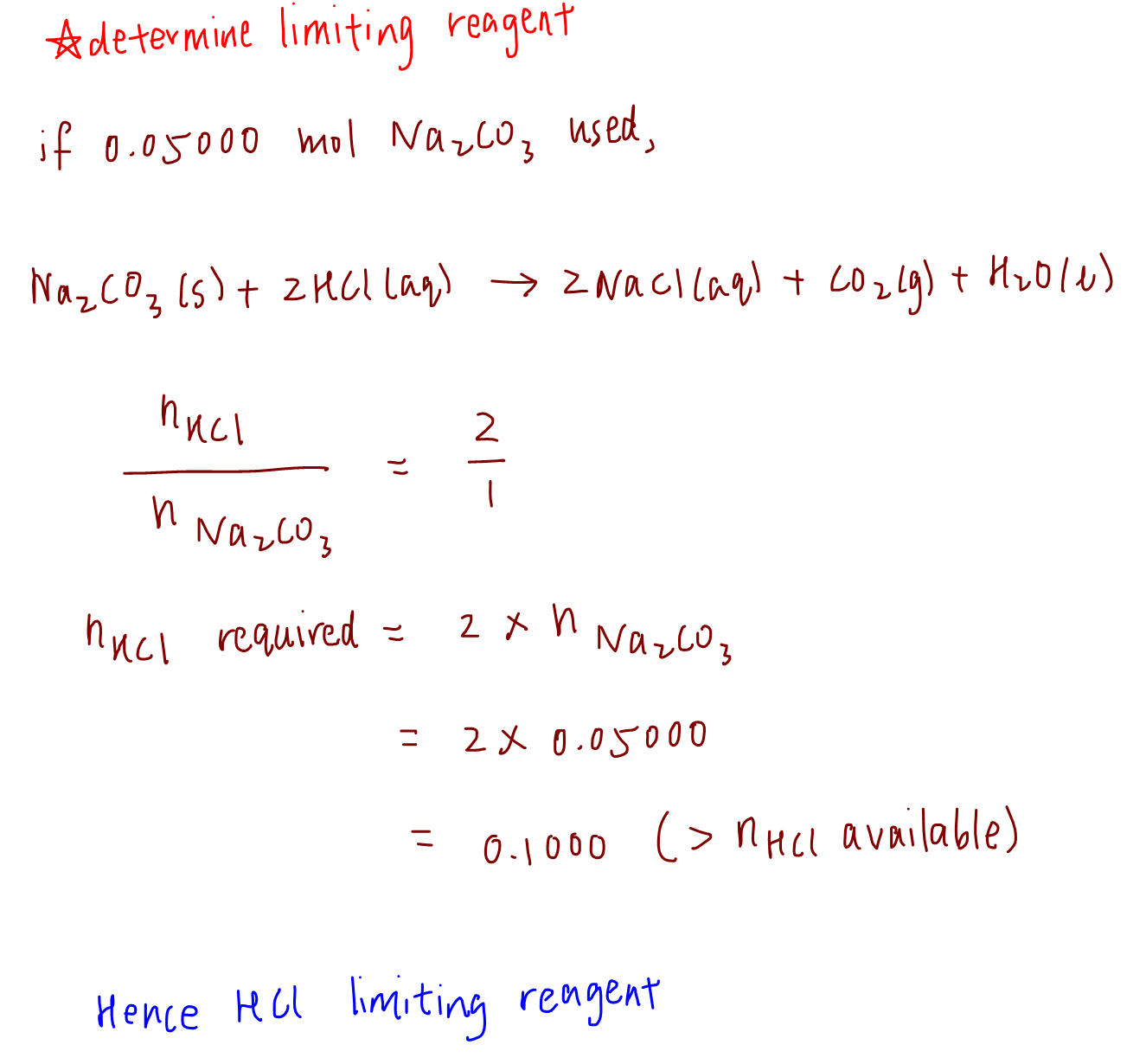CHEM 101: Dimensional Analysis Limiting Reagent, Theoretical Yield, Percent Yield, Excess Reactant 2 - YouTube

SOLVED: 0/1point Step 3: Use the moles of Limiting reactant and the balanced chemical reaction to perform stoichiometric calculation What is the theoretical yield in grams of HzO? 2Hz (g) + 02 (
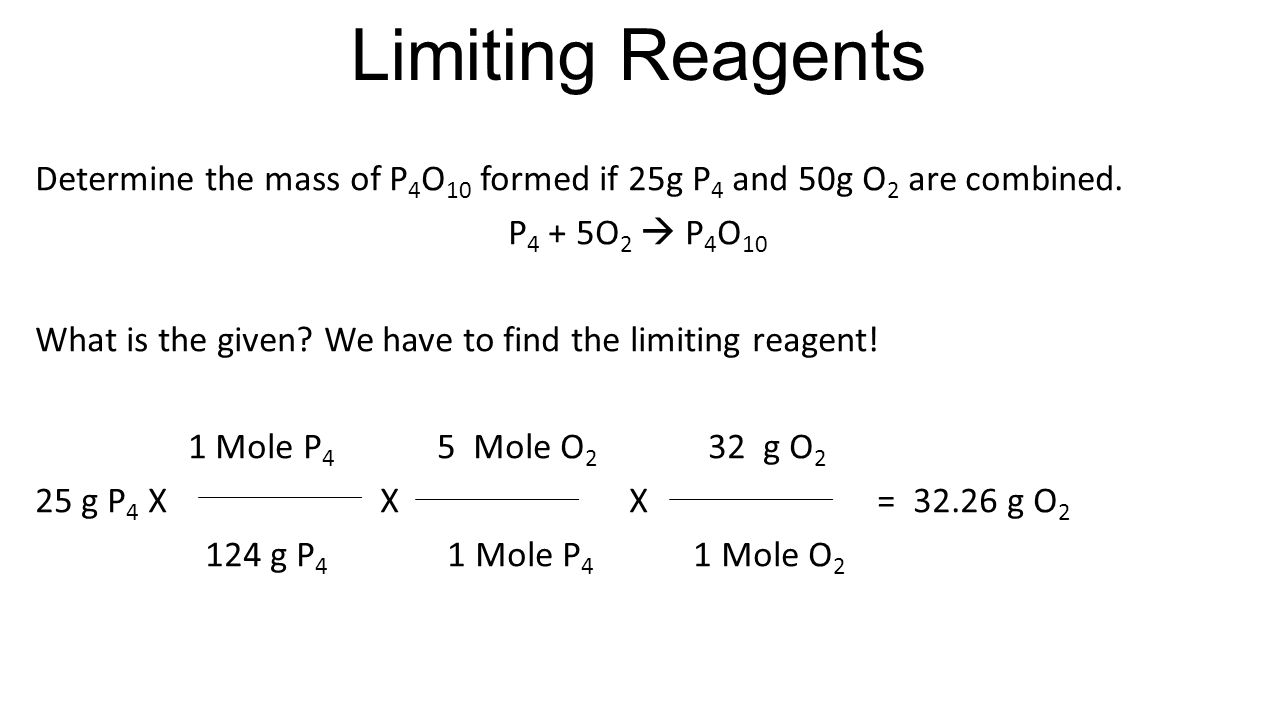
Limiting Reagents Determine the mass of P 4 O 10 formed if 25g P 4 and 50g O 2 are combined. P 4 + 5O 2 P 4 O 10 What
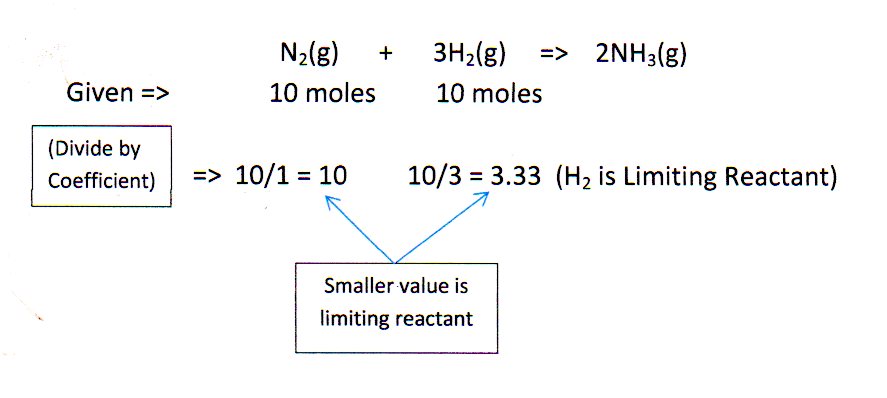
N_2 + 3H_2 -> 2NH_3. What is the limiting reactant if you start the reaction with 10.0 moles of each reactant? | Socratic

How to Find Limiting Reactant (Quick & Easy) Examples, Practice Problems, Practice Questions - YouTube

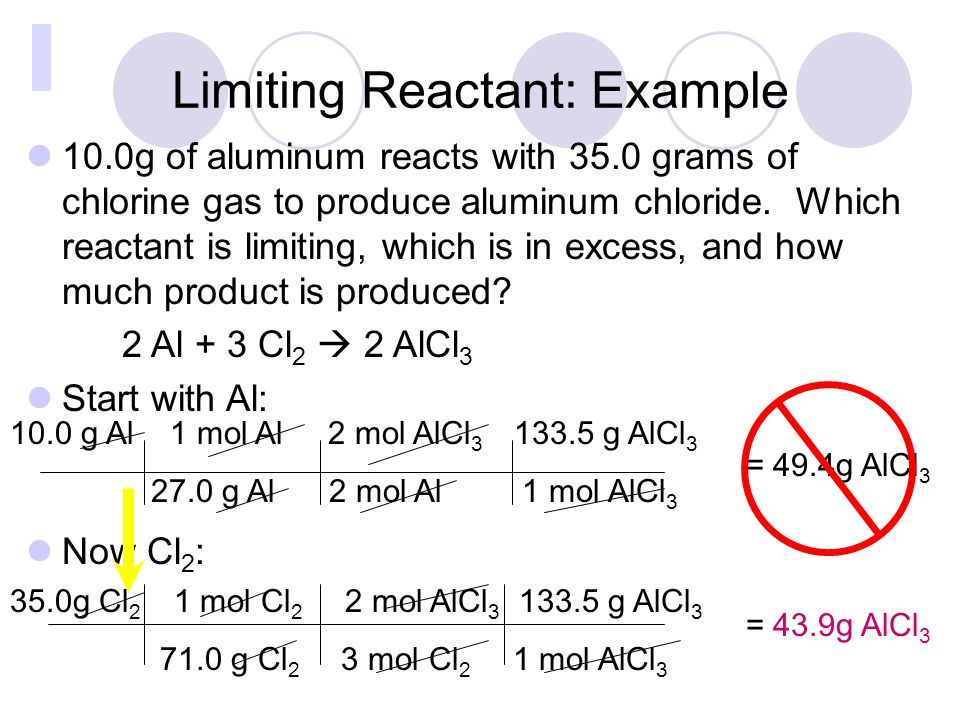


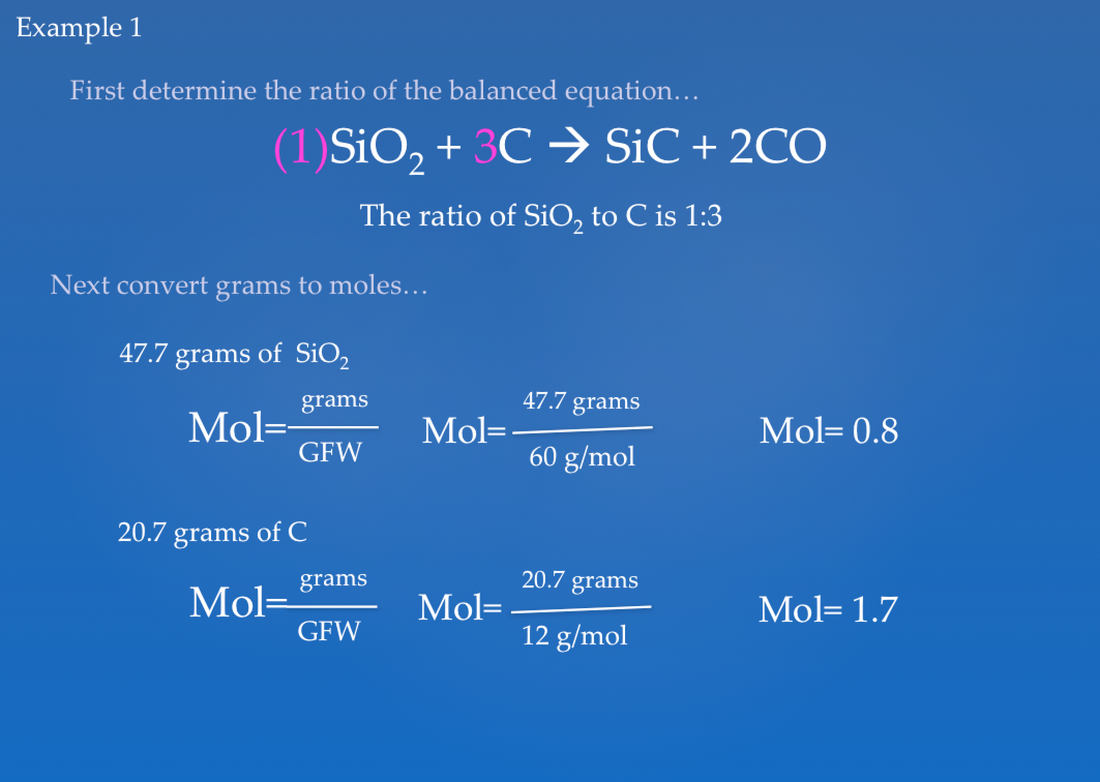


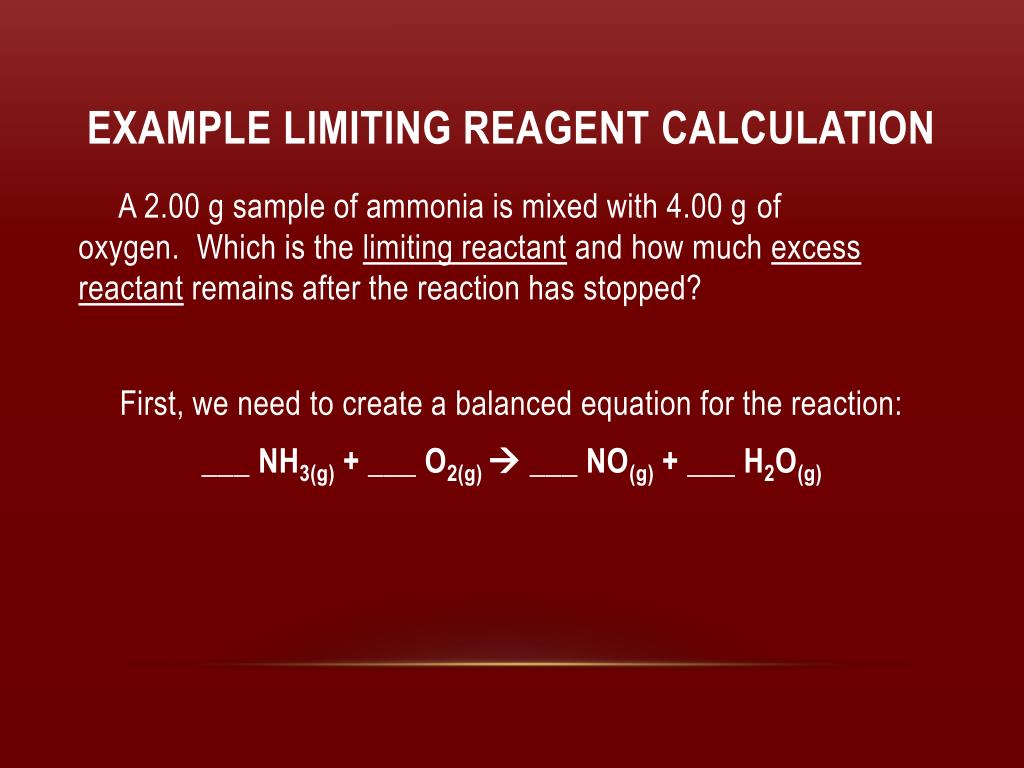

![Solved [Tutorial: Limiting/Excess reactant] This question | Chegg.com Solved [Tutorial: Limiting/Excess reactant] This question | Chegg.com](https://media.cheggcdn.com/study/252/2527ebb6-50c4-438e-aea9-698b27b146bb/image)