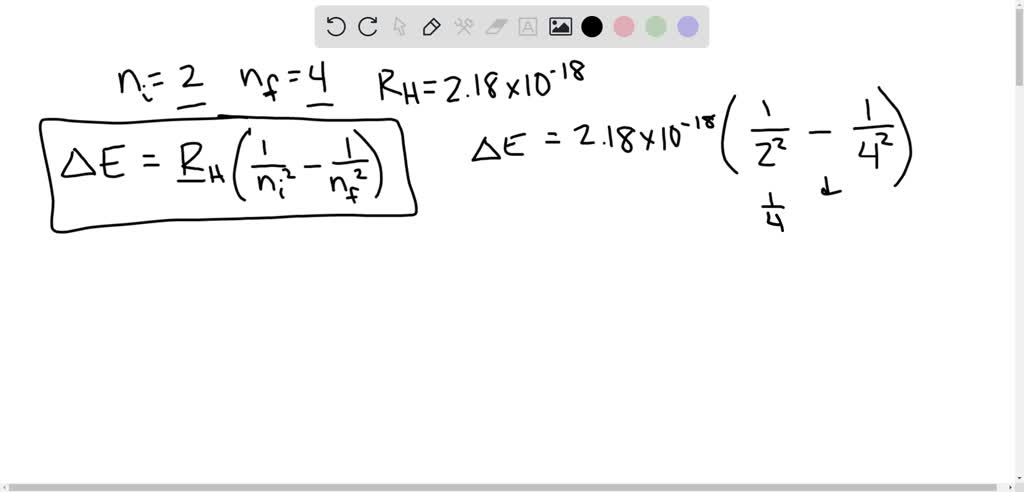
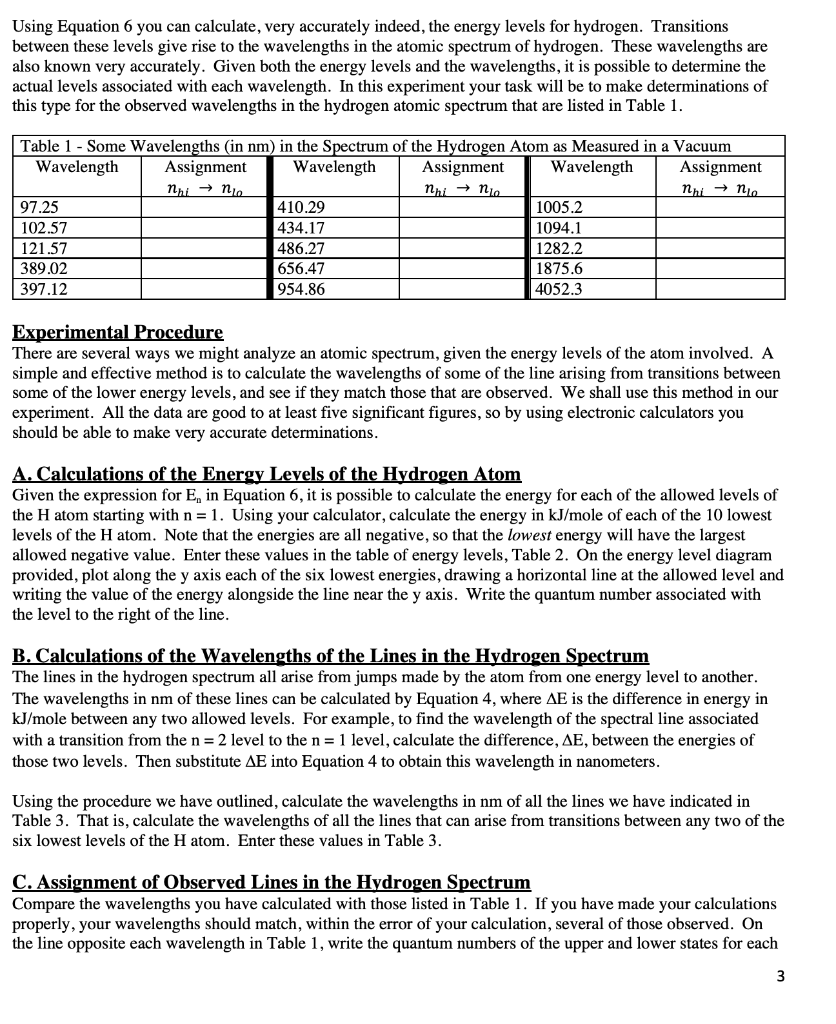
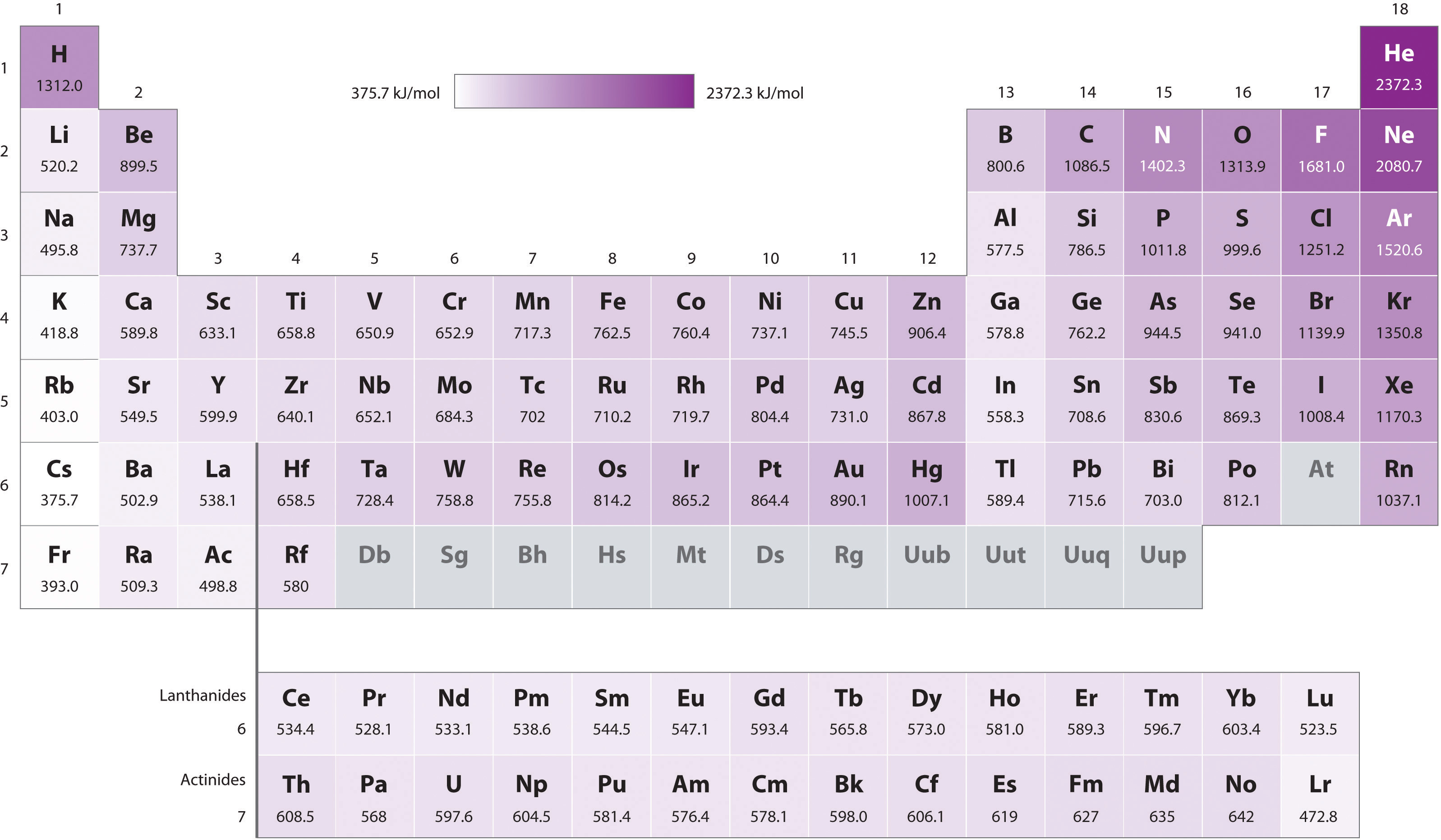
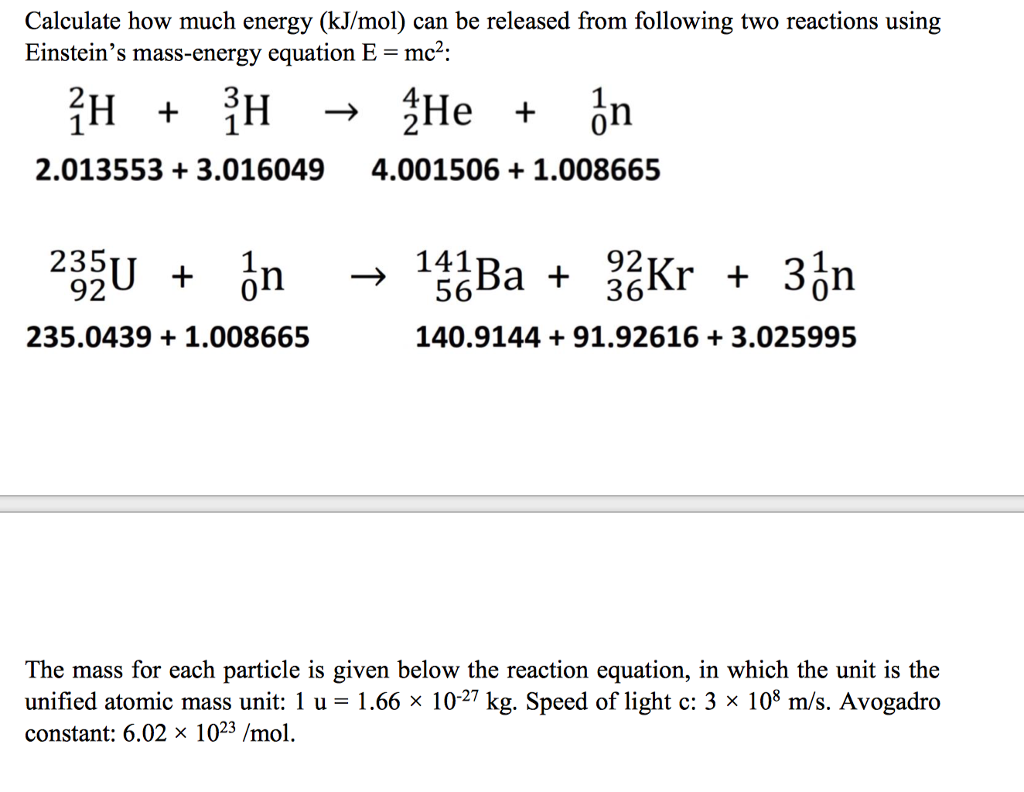
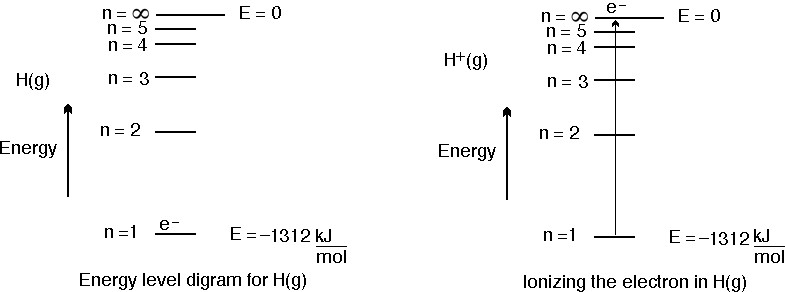
How much energy in KJ/mol is released when an electron makes a transition from n=5 to n=2 in a hydrogen atom? | Homework.Study.com
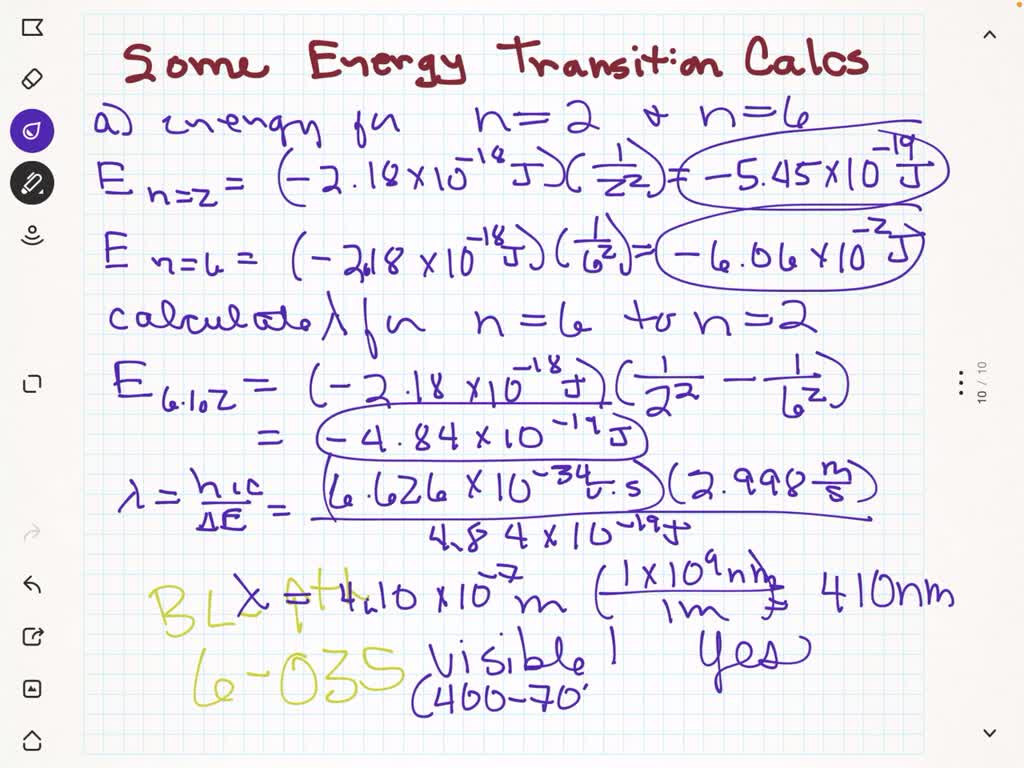
SOLVED: (a) Using Equation 6.5, calculate the energy of an electron in the hydrogen atom when n=2 and when n=6. Calculate the wavelength of the radiation released when an electron moves from
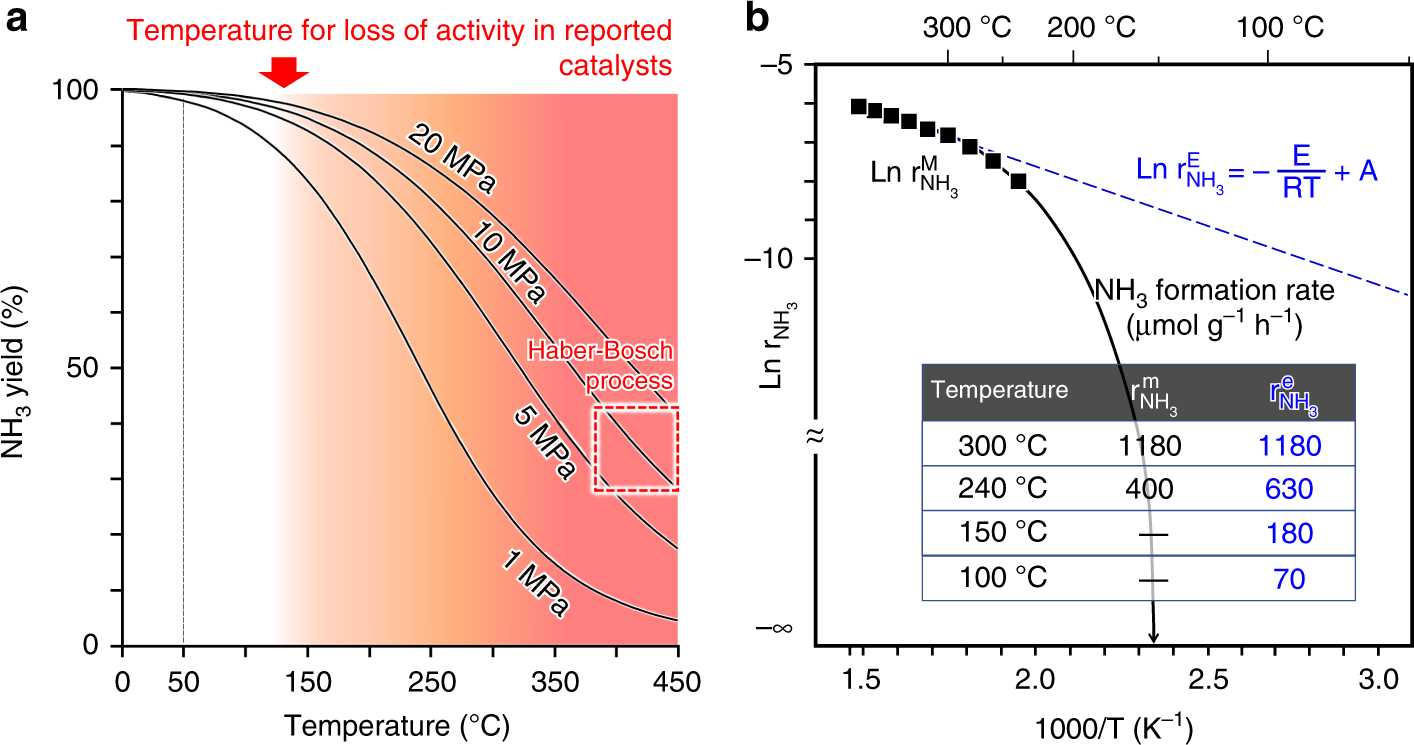
Solid solution for catalytic ammonia synthesis from nitrogen and hydrogen gases at 50 °C | Nature Communications

Calculate the energy in kilojoules per mole of electronic charge accelerated by a potantial of `... - YouTube

The ionization potential of hydrogen is 13.60 eV. Calculate the energy in kJ required to produce 0.1 mole of H^(+) ions. Given, 1 eV = 96.49 kJ mol^(-1) .

Calculate the electronegativity (eV) of chlorine from the bond energy of Cl - F bond (61 kcal mol ^-1 ), F - F bond ( 38 kcal mol ^-1 ) and Cl -