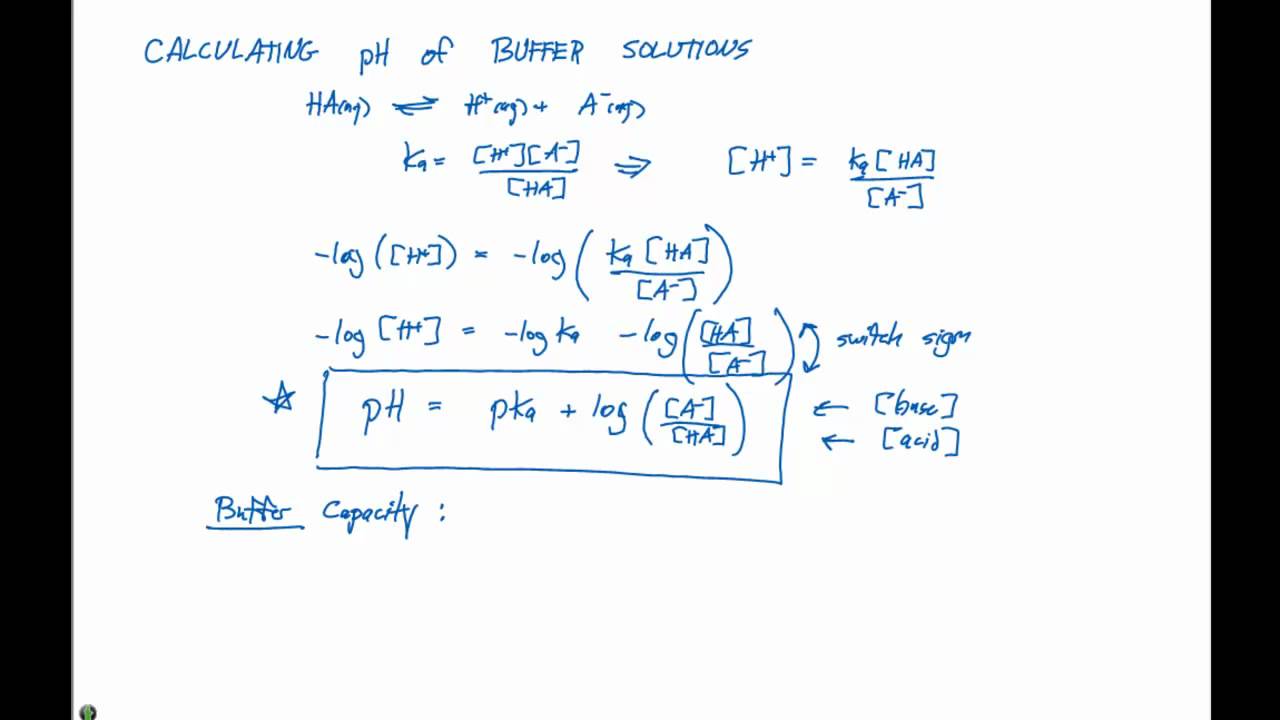
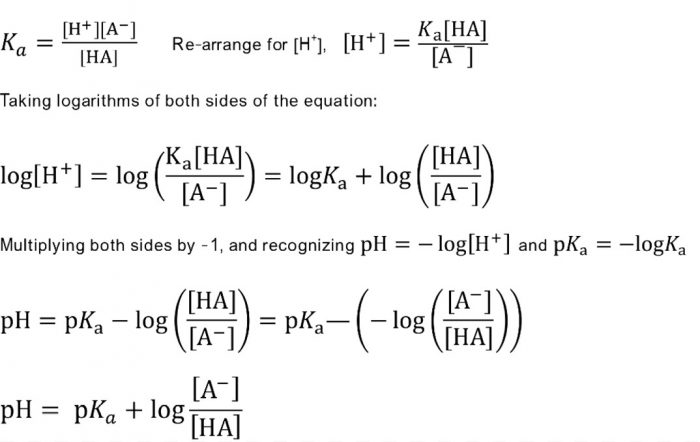
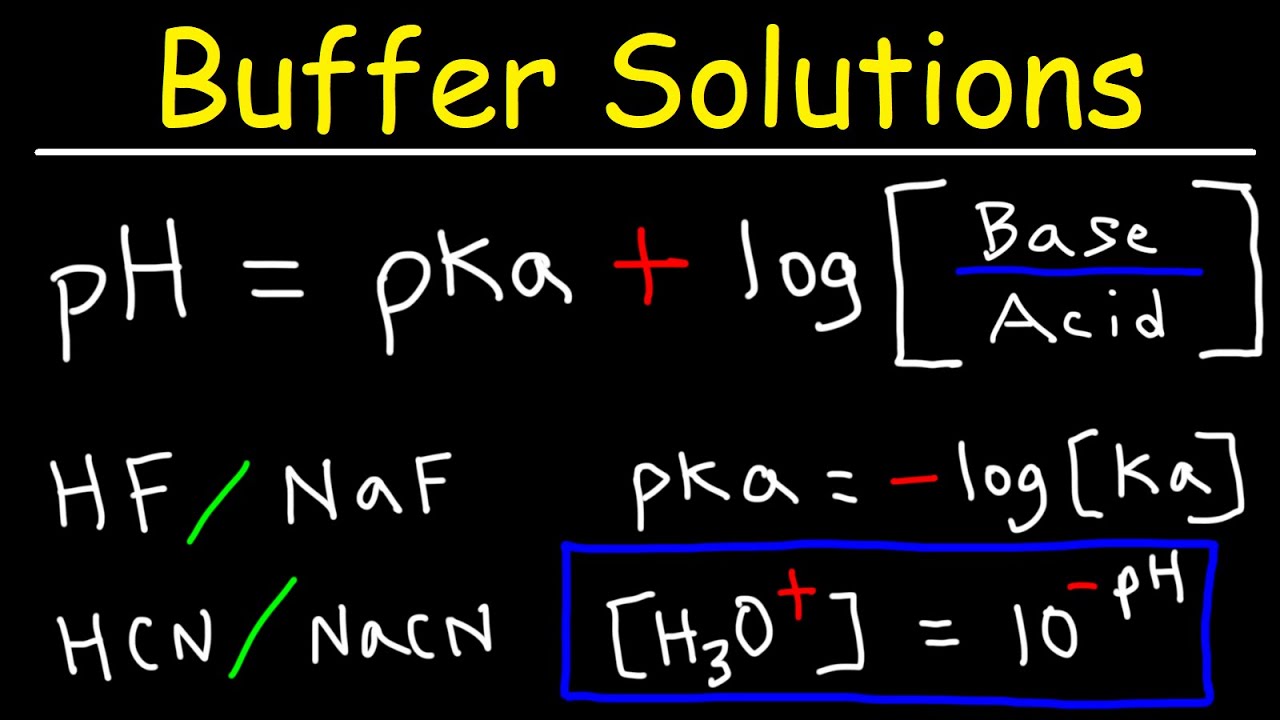
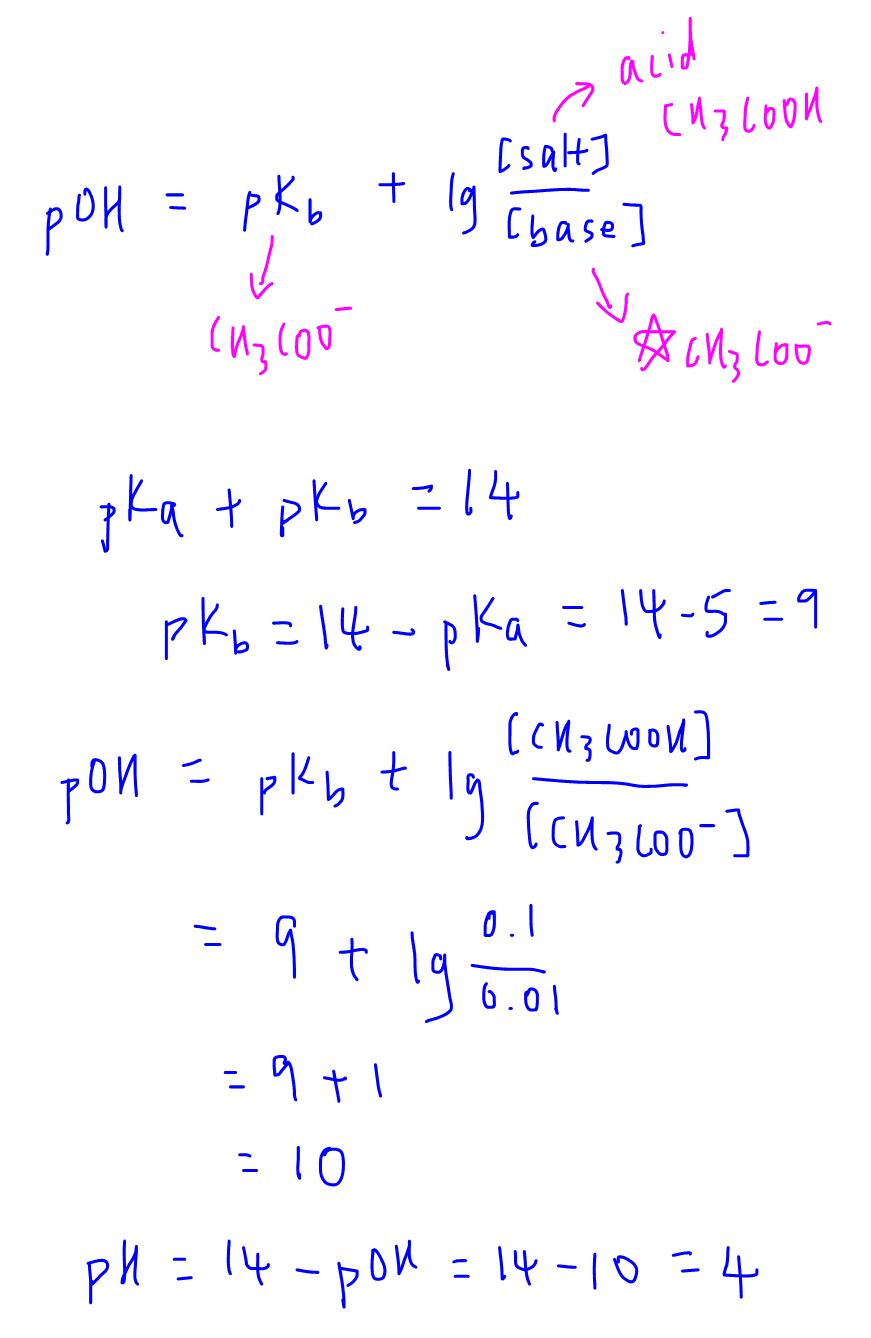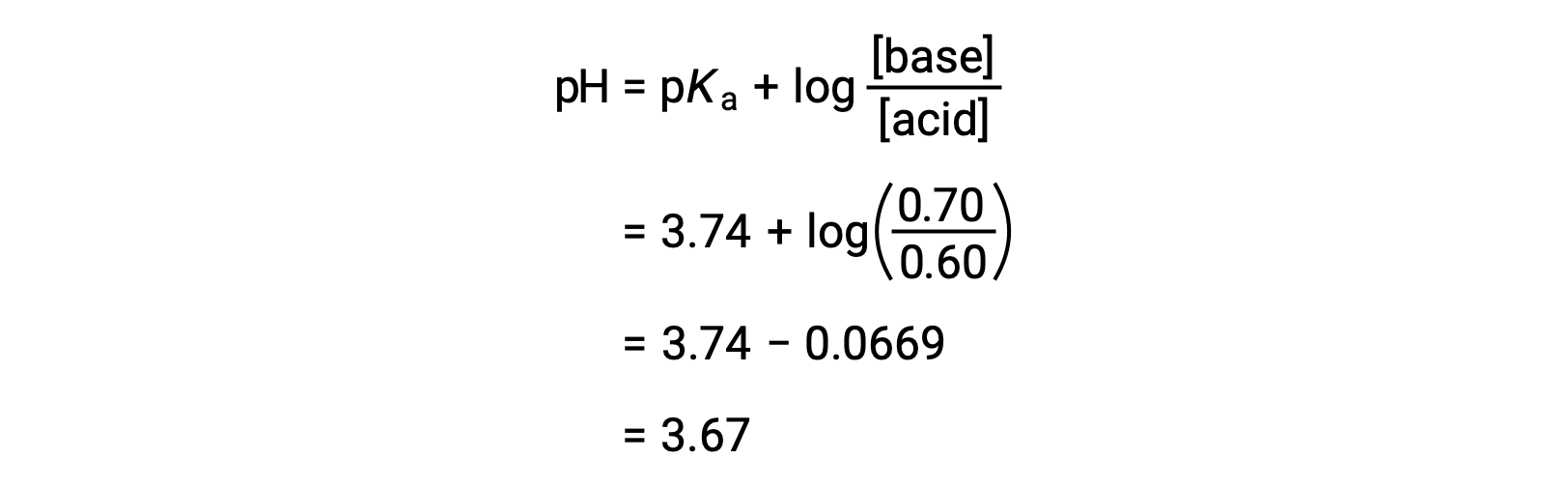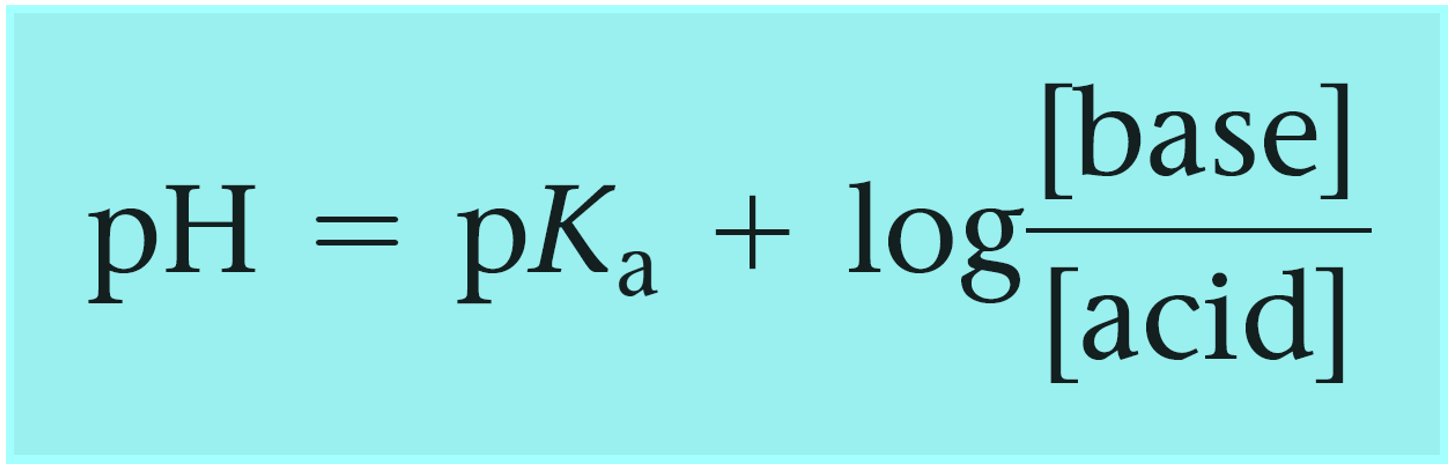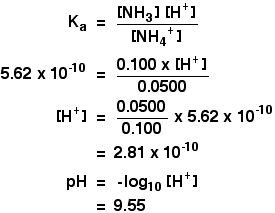Calculate the pH of a buffer prepared by mixing 300 cc of 0.3 M NH3 and 500 cc of 0.5 M NH4Cl . Kb for NH3 = 1.8 × 10^-5

Calculate the PH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 ml of an aqueous solution containing 150 ml of 1m HCL . ka for HCO^-3 = 5.63 x 10 - 11
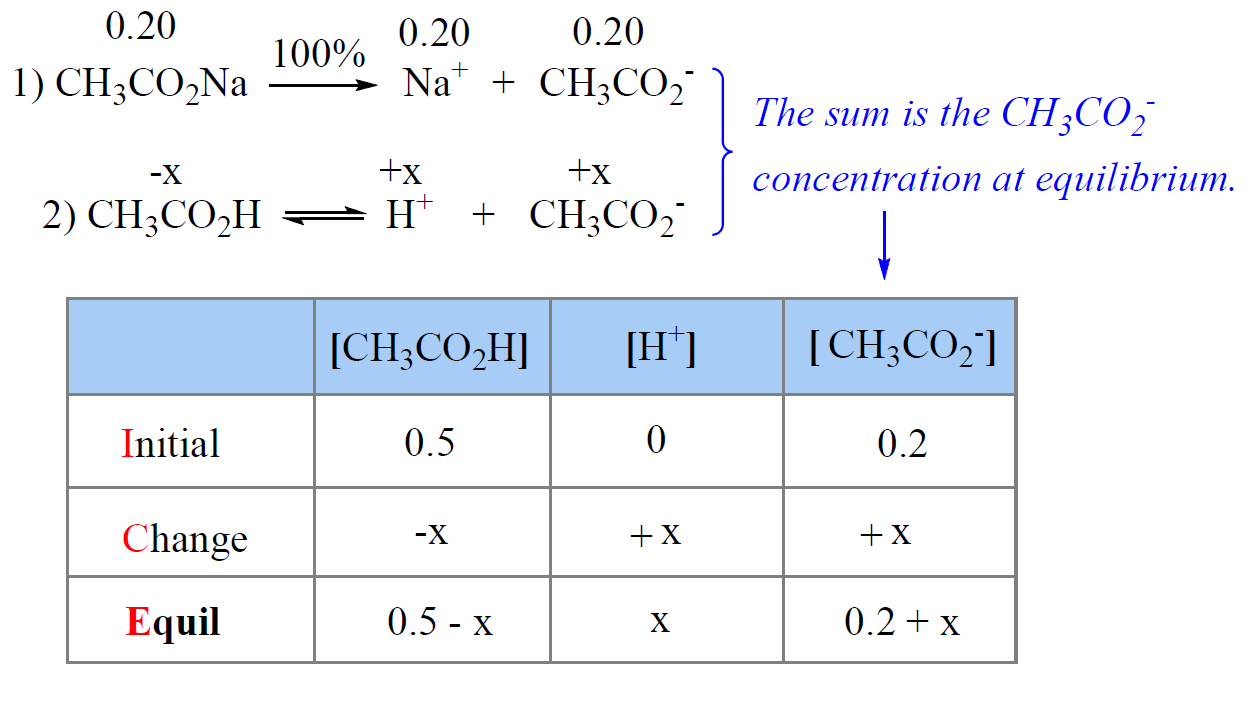
SOLVED: Calculating the Effect of Added H3O+ and OH- on Buffer pH 1. Calculate the pH of a buffer solution (a) consisting of 0.50 M HC2H3O2 and 0.50 M NaC2H3O2 Ka =

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com
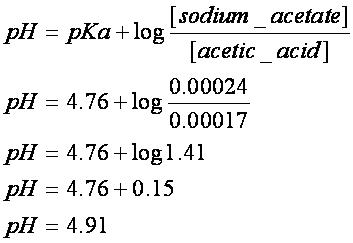
pH calculations and more in fundamentals of pharmaceutics. : Calculate pH of 100 ml buffer solution containing 0.1 g acetic acid and 0.2 g sodium actetate.
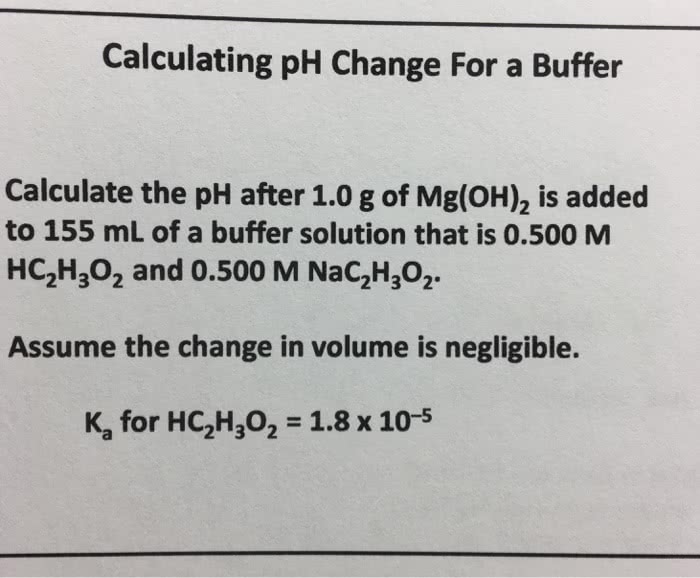
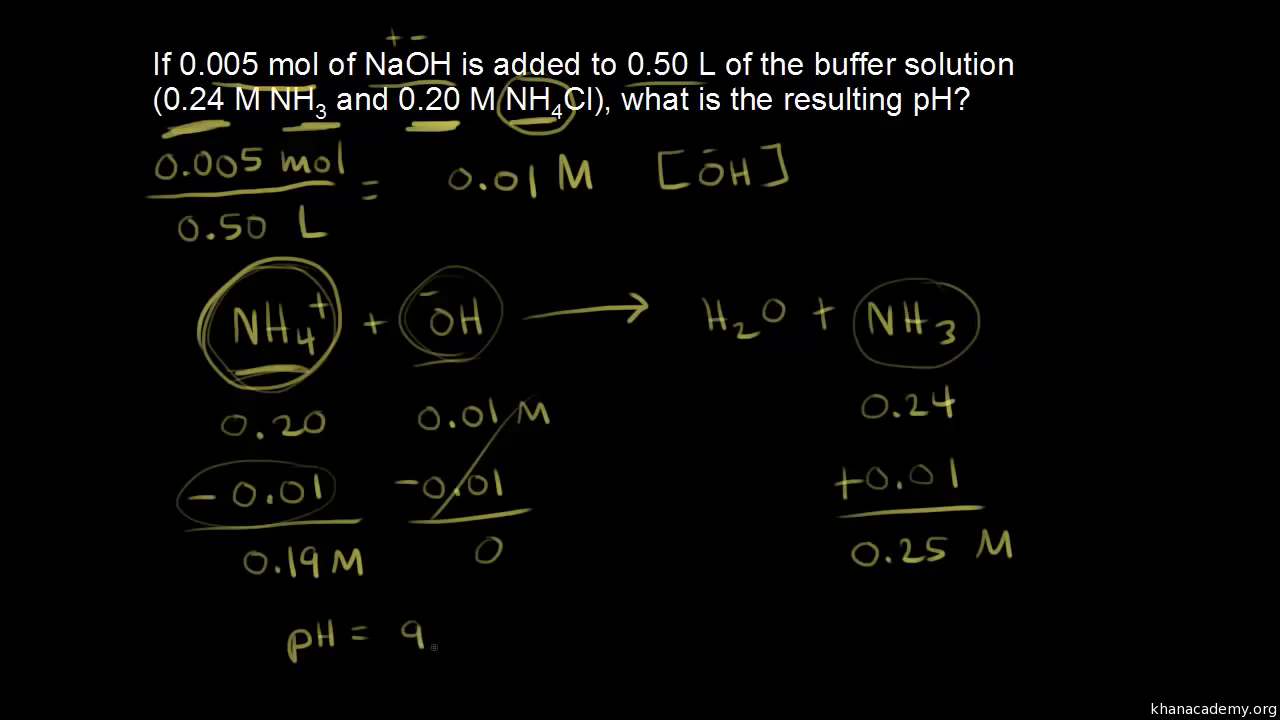
OneClass: Calculating pH Change For a Buffer Calculate the pH after 1.0 g of Mg(OH)_2 is added to 155...
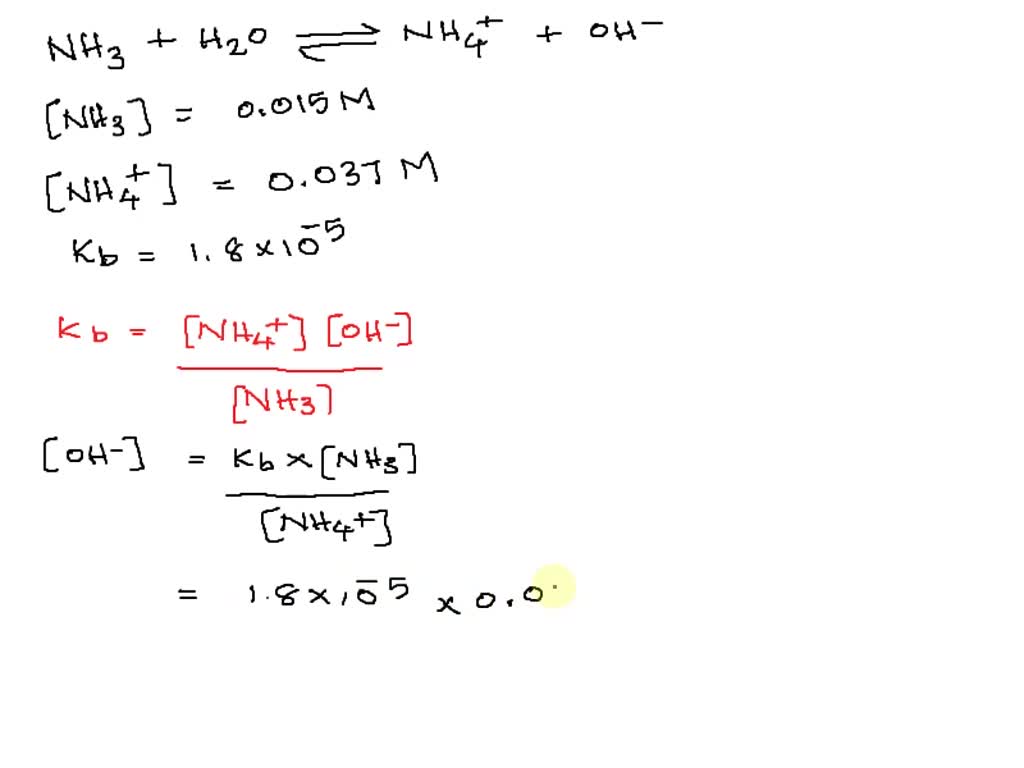
SOLVED: Calculate the pH of a buffer solution consisting of 0.051 M NH3 and 0.037 M NH4+. The Kb for NH3 = 1.8 x 10-5.
![Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ] Calculate pH of a buffer prepared by adding 10 mL of 0.10 M acetic acid to 20 mL of 0.1 M sodium acetate. [pKa (CH3COOH) = 4.74 ]](https://d1hhj0t1vdqi7c.cloudfront.net/v1/dDlCNVZnUE9URzQ=/sd/)